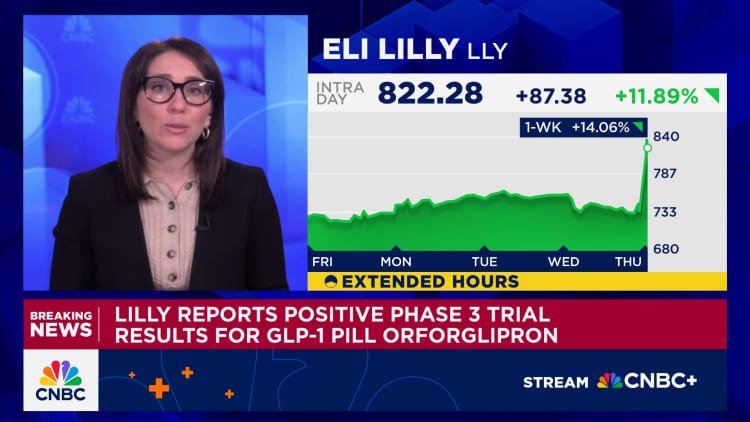
Eli Lilly has announced encouraging results from the first of several late-stage trials of its daily obesity pill, orforglipron. The pill achieved the company’s main objectives by effectively reducing both blood sugar and body weight in patients with Type 2 diabetes. The trial’s outcome brings orforglipron one step closer to becoming a convenient, needle-free alternative in the expanding weight loss and diabetes treatment market. Given its ease of manufacturing and administration, the oral format could provide Eli Lilly with a significant advantage over competitors.
The highest dose of orforglipron led to an average weight reduction of 7.9%, equivalent to about 16 pounds after 40 weeks. Importantly, there was no sign of weight loss plateauing by the end of the trial, indicating potential for continued weight reduction. While patients with Type 2 diabetes generally lose less weight than those without the condition, this result remains notable.
Side effects were mainly gastrointestinal, with 14% experiencing vomiting, 16% reporting nausea, and 26% experiencing diarrhea. Around 8% of participants on the highest dose discontinued treatment due to these effects, a figure slightly below some analysts’ expectations. The side effects were largely mild to moderate.
The pill helped reduce hemoglobin A1c levels — a key measure of blood sugar — by 1.3% to 1.6% across various doses. Though this fell short of some expectations, which projected reductions as high as 2.1%, the results still indicate meaningful glucose control compared to the 0.1% drop observed with a placebo.
Orforglipron is part of a new generation of incretin-based medications, which mimic gut hormones to reduce appetite and regulate blood sugar. Unlike peptide-based alternatives such as Ozempic or Rybelsus, Eli Lilly’s pill is non-peptide, making it easier for the body to absorb and eliminating certain dietary restrictions.
The company is currently running seven late-stage trials — five focused on diabetes and two on obesity. It plans to seek regulatory approval for obesity treatment by the end of the year and for diabetes in 2026. If approved, the pill could ease pressure on the supply of injectable GLP-1 drugs and expand treatment accessibility. With Eli Lilly reportedly years ahead of rival companies developing oral GLP-1 medications, orforglipron may strengthen the company’s leadership in a market projected to exceed $150 billion annually by the early 2030s.
READ MORE: